Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
DESCRIPTION
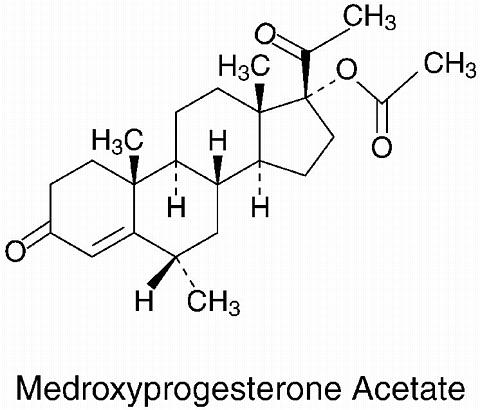
LUNELLE™ Monthly Contraceptive Injection contains medroxyprogesterone acetate and estradiol cypionate as its active ingredients. The chemical name for medroxyprogesterone acetate is pregn-4-ene-3,20-dione,17-(acetyloxy)-6-methyl-(6(alpha))-. The empirical formula is C
24
H
34
O
4
and its molecular weight is 386.53. Medroxyprogesterone acetate is a white to off-white, odorless crystalline powder that is stable in air and melts between 200°C and 210°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and practically insoluble in water. The chemical name for estradiol cypionate is estra-1,3,5,(10)-triene-3,17-diol,(17(beta))-,17-cyclopentanepropanoate. Estradiol cypionate is a white to off-white crystalline powder that melts between 149°C and 153°C. It is soluble in alcohol, acetone, chloroform, and dioxane; sparingly soluble in vegetable oils; and practically insoluble in water. The empirical formula is C
26
H
36
O
3
and its molecular weight is 396.57. The structural formulas for these ingredients are represented below:
LUNELLE™ Monthly Contraceptive Injection is available as a 0.5 mL aqueous suspension and contains 25 mg medroxyprogesterone acetate and 5 mg estradiol cypionate. Inactive ingredients are 0.9 mg methylparaben, 14.28 mg polyethylene glycol, 0.95 mg polysorbate 80, 0.1 mg propylparaben, 4.28 mg sodium chloride, and sterile water for injection.
CLINICAL PHARMACOLOGY
LUNELLE™ Monthly Contraceptive Injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension) when administered at the recommended dose to women every month inhibits the secretion of gonadotropins, which, in turn, prevents follicular maturation and ovulation. Although the primary mechanism of this action is inhibition of ovulation, other possible mechanisms of action include thickening and a reduction in volume of cervical mucus (which decrease sperm penetration) and thinning of the endometrium (which may reduce the likelihood of implantation).
Pharmacokinetics
Steady-state pharmacokinetic parameters of medroxyprogesterone acetate (MPA) and 17(beta)-estradiol (E
2
), the parent active moiety of estradiol cypionate (E
2
C), following the third monthly injection of LUNELLE™ Monthly Contraceptive Injection are shown in Table 1.
Table 1. Pharmacokinetic Parameters of Medroxyprogesterone Acetate (MPA)
and Estradiol (17(beta)-E
2
)
after the 3rd Monthly Injection of LUNELLE™ Monthly Contraceptive Injection
in 14 Surgically Sterile Women
|
|
C
max
(ng/mL)
|
T
max
(day)
|
AUC
0-28
(ng·day/mL)
|
AUC
0-(infinity)
(ng·day/mL)
|
t
1/2
(day)
|
|
MPA
|
Mean |
1.25
|
3.5
|
21.51
|
33.65
|
14.7
|
|
Min
|
0.94
|
1.0
|
14.44
|
22.02
|
6.2
|
|
Max
|
2.17
|
10.0
|
27.00
|
49.09
|
36.0
|
|
17(beta)-E
2
|
Mean |
0.25
|
2.1
|
2.74
|
2.99
|
8.4
|
|
Min
|
0.14
|
1.0
|
1.65
|
1.65
|
2.6
|
|
Max
|
0.48
|
7.0
|
3.56
|
3.89
|
20.4
|
|
|
|
Absorption
Absorption of MPA and E
2
from the injection site is prolonged after an intramuscular injection of LUNELLE™ Monthly Contraceptive Injection. The time to maximum plasma concentration (T
max
) typically occurs within 1 to 10 days postinjection for MPA and 1 to 7 days postinjection for E
2
. The peak concentrations (C
max
) generally range from 0.94 to 2.17 ng/mL for MPA and from 140 to 480 pg/mL for E
2
. The PK profile of E
2
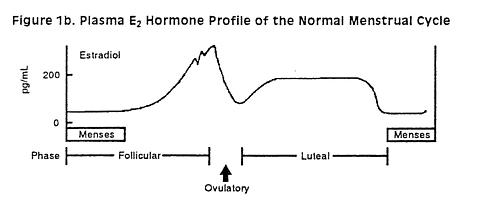
following administration of LUNELLE™ Monthly Contraceptive Injection is shown in Figure 1a.
Berne RM, Levy MN, 1988.
Effect of Injection Site:
AUC
0-28
for MPA values were statistically significantly higher following injection of LUNELLE™ Monthly Contraceptive Injection into the arm as compared to the anterior thigh (average increase was approximately 25%). The mean MPA C
max
was higher but not statistically significant (average increase 6 to 12%) when LUNELLE™ Monthly Contraceptive Injection was injected into the arm compared with the C
max
observed after injection into the hip or the anterior thigh. However, the average MPA trough (C
min
) concentrations and the half-lives were comparable for the three injection sites. E
2
concentrations were not measured.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin; no binding of MPA occurs with sex-hormone-binding globulin (SHBG). Estrogens circulate in blood bound to albumin, SHBG, (alpha)1-glycoproteins, and transcortin. Estradiol is primarily bound to SHBG and albumin and approximately 3% remains unbound. Unbound estrogens are known to modulate pharmacologic response.
Metabolism:
MPA is extensively metabolized. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in numerous derivatives. E
2
C undergoes ester hydrolysis after intramuscular injection of LUNELLE™ Monthly Contraceptive Injection, releasing the parent, active compound E
2
. Exogenously delivered or endogenously derived E
2
is primarily metabolized to estrone and estriol, both of which are metabolized to their sulfate and glucuronide forms.
Elimination
Residual MPA concentrations at the end of a monthly injection of LUNELLE™ Monthly Contraceptive Injection are generally below 0.5 ng/mL, consistent with its apparent elimination half-life of 15 days. Most MPA metabolites are excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates. Following the peak concentration, serum E
2
levels typically decline to 100 pg/mL by day 14 and are consistent with the apparent elimination half-life of 7 to 8 days. Estrogen metabolites are primarily excreted in the urine as glucuronides and sulfates.
Return of Ovulation:
Return of ovulation correlated to some extent with MPA AUC
0-84
days. Additionally, body weight and site of injection affected the AUC of MPA. AUC
0-28
values are significantly higher when LUNELLE™ Monthly Contraceptive Injection is injected into the arm compared to the anterior thigh muscle and into women with BMI </=28 kg/m
2
compared to those with BMI >28 kg/m
2
. Consequently, return of ovulation may be delayed in women with BMI </=28 kg/m
2
who receive an injection in the arm.
Pharmacokinetics in Subpopulations
Race:
The pharmacokinetics of MPA and E
2
has been evaluated in different populations in separate studies. With the exception of one study in Thai women that demonstrated relatively higher C
max
and shorter T
max
values indicating more rapid absorption of both MPA and E
2
, the pharmacokinetics of MPA and E
2
after the administration of LUNELLE™ Monthly Contraceptive Injection were similar in women from various ethnic backgrounds. Although pharmacokinetic differences were observed, the contraceptive efficacy was similar among all women of all ethnic backgrounds studied. Following discontinuation, ovulation returned earlier in Thai women.
Pediatric
Safety and efficacy of LUNELLE™ Monthly Contraceptive Injection have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under 16 years of age and users 16 years of age and older. Use of this product before menarche is not indicated.
Geriatric
LUNELLE™ Monthly Contraceptive Injection is intended for use in healthy women desiring contraception; studies in geriatric women have not been conducted.
Effect of Body Weight:
No dosage adjustment is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA was assessed in a subset of women (n = 77, body mass index ranged from 18 to 45.5 kg/m
2
) enrolled in a Phase 3 trial. AUC
0-28
values for MPA were significantly higher in thinner women with body mass index </=28 kg/m
2
(average increase was approximately 20%) when compared to that in heavier women with body mass index >28 kg/m
2
. The mean MPA C
max
was higher (average increase 42%) in thin/normal women with body mass index </=28 kg/m
2
compared with heavier women with body mass index >28 kg/m
2
. The range of MPA trough (C
min
) concentrations and the half-lives were comparable for both groups.
Hepatic Insufficiency:
No formal studies have evaluated the effect of hepatic disease on the disposition of LUNELLE™ Monthly Contraceptive Injection. However, steroid hormones may be poorly metabolized in patients with impaired liver function. (See CONTRAINDICATIONS .)
Renal Insufficiency:
No formal studies have evaluated the effect of renal disease on the pharmacokinetics of LUNELLE™ Monthly Contraceptive Injection. However, since both steroidal components of LUNELLE™ Monthly Contraceptive Injection are almost exclusively eliminated by hepatic metabolism, no dosage adjustment is necessary in women with renal dysfunction.
Drug-Drug Interactions
No formal drug-drug interaction studies were conducted with LUNELLE™ Monthly Contraceptive Injection. Aminoglutethimide administered concomitantly with LUNELLE™ Monthly Contraceptive Injection may significantly depress the serum concentrations of MPA. Users of LUNELLE™ Monthly Contraceptive Injection should be warned of the possibility of decreased efficacy with the use of this or any related drugs. (See PRECAUTIONS , DRUG INTERACTIONS .)
CLINICAL STUDIES
LUNELLE™ Monthly Contraceptive Injection has been studied for safety and efficacy in various comparative and introductory clinical trials around the world. One US study was performed with the goal of describing bleeding patterns in women using LUNELLE™ Monthly Contraceptive Injection compared to women using a standard oral contraceptive product. The group of LUNELLE™ Monthly Contraceptive Injection users in this study was 67.9% White, 15.5% Hispanic, 13.6% Black, 2.4% Asian, and 0.6% other.
In the clinical trials, reported 12-month pregnancy rates have been low (</=0.2%). Due to certain limitations of the available data (loss to follow-up, lack of pregnancy testing, use of barrier contraceptive products, and concomitant medications, etc.), a precise estimate of the failure rate is not possible, but is likely in the range of 0.1 to 1%.
The bleeding pattern over one year of use for LUNELLE™ Monthly Contraceptive Injection was examined in the US trial. Bleeding patterns during the last three months (months 9-12) of LUNELLE™ Monthly Contraceptive Injection use were compared with a concurrent group of standard oral contraceptive users. During this last three-month reference period, 58.6% of women using LUNELLE™ Monthly Contraceptive Injection experienced altered bleeding patterns (compared to 23.7% in the comparison group). See also WARNINGS , BLEEDING IRREGULARITIES. The one-year Life Table bleeding-related discontinuation rate for LUNELLE™ Monthly Contraceptive Injection was 6.1% for 782 participants in a US trial of up to 15 months duration. Bleeding patterns did not predict discontinuation from this large clinical trial.
Bleeding data from the US trial was re-analyzed based on injection intervals of 23 to 33 days. During the first injection interval, withdrawal bleeding lasted for more than 7 days in 42% of women, including 16% whose bleeding exceeded 10 days. The remaining 58% experienced bleeding for 7 days or less. Withdrawal bleeding began between days 20 and 25 (median 21) after initial injection in 48% of women using LUNELLE™ Monthly Contraceptive Injection.
At the end of one year of treatment, withdrawal bleeding lasted for more than 7 days in 29% of women, including 7% whose bleeding exceeded 10 days. The remaining 71% experienced bleeding 7 days or less in duration. Fifty percent of patients experienced withdrawal bleeding that began within 21-25 days (median 22) after their previous injection.
In any given injection interval, approximately 75% of women experienced a single withdrawal bleeding episode, without additional breakthrough bleeding or spotting, during that interval. In any given injection interval, approximately 15% of women experienced no bleeding and 10% experienced bleeding or spotting at various times in that injection interval.
In the US trial, weight gain was the most common adverse event leading to discontinuation of LUNELLE™ Monthly Contraceptive Injection (5.7% LUNELLE™ Monthly Contraceptive Injection group vs. 0.9% in the oral contraceptive comparator group). Weight change over 12 months in the LUNELLE™ Monthly Contraceptive Injection group ranged from 48 pounds lost to 49 pounds gained. Mean body weight change in the LUNELLE™ Monthly Contraceptive Injection group was a gain of 4 pounds after 13 injections and a gain of 5 pounds after 15 injections. Wide variability in individual weight gain or loss was observed; however, an increasing percentage of LUNELLE™ Monthly Contraceptive Injection users exhibited weight change in excess of 10 and 20 pounds with continued treatment. See also PRECAUTIONS , Weight Change.
INDICATIONS AND USAGE
LUNELLE™ Monthly Contraceptive Injection is indicated for the prevention of pregnancy.
The efficacy of LUNELLE™ Monthly Contraceptive Injection is dependent on adherence to the recommended dosage schedule (e.g., intramuscular injections every 28 to 30 days, not to exceed 33 days). To ensure that LUNELLE™ Monthly Contraceptive Injection is not administered inadvertently to a pregnant woman, the first injection should be given during the first 5 days of a normal menstrual period. LUNELLE™ Monthly Contraceptive Injection should be administered no earlier than 4 weeks after delivery if not breastfeeding or 6 weeks after delivery if breastfeeding (see NURSING MOTHERS ).
Several clinical trials of LUNELLE™ Monthly Contraceptive Injection have reported 12-month failure rates of <1% by Life Table analysis (see also CLINICAL STUDIES ). Pregnancy rates for various contraceptive methods are typically reported for the first year of use and are shown in Table 2.
Table 2. Percentage of Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year: United States
|
|
% of Women
Experiencing
an Unintended
Pregnancy within
the First Year
of Use
|
% of
Women
Continuing
Use at
1 Year
3
|
|
|
Typical
Use
1
|
Perfect
Use
2
|
|
|
|
85
|
85
|
|
|
|
26
|
6
|
40
|
|
|
25
|
|
63
|
|
Calendar
|
|
9
|
|
|
|
|
3
|
|
|
|
|
2
|
|
|
Post-ovulation
|
|
1
|
|
|
|
|
|
|
|
Parous Women
|
40
|
26
|
42
|
|
Nulliparous Women
|
20
|
9
|
56
|
|
|
|
|
|
|
Parous Women
|
40
|
20
|
42
|
|
Nulliparous Women
|
20
|
9
|
56
|
|
|
20
|
6
|
56
|
|
|
19
|
4
|
|
|
|
|
|
|
|
Female (Reality)
|
21
|
5
|
56
|
|
Male
|
14
|
3
|
61
|
|
|
5
|
|
71
|
|
Progestin only
|
|
0.5
|
|
|
Combined
|
|
0.1
|
|
|
IUD
|
|
|
|
|
Progesterone T
|
2.0
|
1.5
|
81
|
|
Copper T 380A
|
0.8
|
0.6
|
78
|
|
LNg 20
|
0.1
|
0.1
|
81
|
|
Depo-Provera
|
0.3
|
0.3
|
70
|
|
Norplant and
|
|
|
|
|
Norplant-2
|
0.05
|
0.05
|
88
|
|
|
0.5
|
0.5
|
100
|
|
|
0.15
|
0.10
|
100
|
Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%.
9
Lactational Amenorrhea Method: LAM is a highly effective, temporary method of contraception.
10
|
Adapted from Hatcher et al., 1998.
|
1
Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
|
2
Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
|
3
Among couples attempting to avoid pregnancy, the percentage who continue to use a method for 1 year.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CONTRAINDICATIONS
The information contained in this package insert is based not only on information specific to LUNELLE™ Monthly Contraceptive Injection, but also on studies carried out in women who used injectable progestin-only contraceptives (medroxyprogesterone acetate) or oral contraceptives with higher doses of both estrogens and progestogens than those in common use today. The effect of long-term use of hormonal contraceptives with formulations having lower doses of both estrogens and progestogens remains to be determined.
LUNELLE™ Monthly Contraceptive Injection should not be used in women with any of the following conditions or circumstances.
WARNINGS
|
Cigarette smoking increases the risk of serious cardiovascular side effects from contraceptives containing estrogen. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use LUNELLE™ Monthly Contraceptive Injection should be strongly advised not to smoke.
|
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity, and diabetes.
Practitioners prescribing LUNELLE™ Monthly Contraceptive Injection should be familiar with the following information relating to these risks.
Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among non-users. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and non-users. The attributable risk does provide information about the actual occurrence of a disease in the population. For further information, the reader is referred to a text on epidemiological methods.
-
THROMBOEMBOLIC DISORDERS AND OTHER VASCULAR PROBLEMS
-
Myocardial Infarction
An increased risk of myocardial infarction has been attributed to oral contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current oral contraceptive users has been estimated to be two to six. The risk is very low in women under the age of 30.
Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers over 35 years of age and older and non-smokers over 40 years of age who use oral contraceptives (see Table 3).
Table 3. Circulatory Disease Mortality Rates per 100,000 Women
Years by Age, Smoking Status and Oral Contraceptive Use
|
Age (y)
|
Ever-Users
Non-smokers
|
Ever-Users
Smokers
|
Controls
Non-smokers
|
Controls
Smokers
|
|
15-24
|
0.0
|
10.5
|
0.0
|
0.0
|
|
25-34
|
4.4
|
14.2
|
2.7
|
4.2
|
|
35-44
|
21.5
|
63.4
|
6.4
|
15.2
|
|
45+
|
52.4
|
206.7
|
11.4
|
27.9
|
|
Adapted from Layde PM, Beral V., 1981.
|
|
Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age, and obesity. In particular, some progestogens are known to decrease high density lipoproteins (HDL) cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism. Oral contraceptives have been shown to increase blood pressure among users (see WARNINGS , No. 9). Similar effects on risk factors have been associated with an increased risk of heart disease. LUNELLE™ Monthly Contraceptive Injection must be used with caution in women with cardiovascular disease risk factors.
-
Thromboembolism
An increased risk of thromboembolic and thrombotic diseases associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared with non-users to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization. The risk of thromboembolic disease due to oral contraceptives is not related to length of use and disappears after pill use is stopped.
A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of oral contraceptives. The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions. If feasible, oral contraceptives should be discontinued at least 4 weeks prior to and for 2 weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of thromboembolism, oral contraceptives and other combined hormonal contraceptives such as LUNELLE™ Monthly Contraceptive Injection, should be started no earlier than 4 weeks after delivery.
The clinician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, pulmonary embolism, cerebrovascular disorders, and retinal thrombosis). Should any of these occur or be suspected, LUNELLE™ Monthly Contraceptive Injection should not be readministered.
-
Cerebrovascular Disease
Oral contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and non-users, for both types of strokes, while smoking interacted to increase the risk for hemorrhagic stroke.
The relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension. The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users, and 25.7 for users with severe hypertension. The attributable risk is also greater in older women.
-
Dose-related Risk of Vascular Disease
A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease. A decline in serum HDL has been reported with many progestational agents. A decline in serum HDL has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the type of progestogens used in the contraceptives. The activity and amount of both hormones should be considered in the choice of a hormonal contraceptive.
-
Persistence of Risk of Vascular Disease
There are two studies which have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least 9 years for women 40-49 years who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups. In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least 6 years after discontinuation of oral contraceptives, although excess risk was very small. However, both studies were performed with oral contraceptive formulations containing 50 micrograms or more of estrogen.
-
ESTIMATES OF MORTALITY FROM CONTRACEPTIVE USE
One study gathered data from a variety of sources that have estimated the mortality rate associated with different methods of contraception at different ages (see Table 4). These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risks. The study concluded that with the exception of oral contraceptive users 35 years and older who smoke, and oral contraceptive users 40 years and older who do not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth.
The observation of a possible increase in risk of mortality with age for oral contraceptive users is based on data gathered in the 1970s, but not reported until 1983. However, current clinical practice involves the use of lower estrogen-dose formulations combined with careful restriction of oral contraceptive use to women who do not have the various risk factors listed in this labeling.
Because of these changes in practice and because of some limited new data that suggest the risk of cardiovascular disease with the use of oral contraceptives may now be less than previously observed, the Fertility and Maternal Health Drugs Advisory Committee was asked to review the topic in 1989. The Committee concluded that although cardiovascular disease risk may be increased with oral contraceptive use after age 40 in healthy non-smoking women (even with the newer low-dose formulations), there are also greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures that may be necessary if such women do not have access to effective and acceptable means of contraception. Therefore, the Committee recommended that the benefits of oral contraceptive use by healthy non-smoking women over age 40 may outweigh the possible risks. Women of all ages who take oral contraceptives should take a product which contains the lowest amount of estrogen and
progestogen that is effective.
Table 4. Annual Number of BIrth-Related or Method-Related Deaths Associated with Control of Fertility per
100,000 Non-sterile Women, by Fertility Control Method According to Age
|
|
Range of Ages (years)
|
|
15-19
|
20-24
|
25-29
|
30-34
|
35-39
|
40-44
|
|
|
7.0
|
7.4
|
9.1
|
14.8
|
25.7
|
28.2
|
Oral hormonal contraceptives **
(non-smoker)
|
0.3
|
0.5
|
0.9
|
1.9
|
13.8
|
31.6
|
Oral hormonal contraceptives **
(smoker)
|
2.2
|
3.4
|
6.6
|
13.5
|
51.1
|
117.2
|
|
|
0.8
|
0.8
|
1.0
|
1.0
|
1.4
|
1.4
|
|
|
1.1
|
1.6
|
0.7
|
0.2
|
0.3
|
0.4
|
|
|
1.9
|
1.2
|
1.2
|
1.3
|
2.2
|
2.8
|
|
|
2.5
|
1.6
|
1.6
|
1.7
|
2.9
|
3.6
|
|
Adapted from Ory HW. 1983.
|
* Deaths are birth-related
|
|
|
|
-
CARCINOMA OF THE REPRODUCTIVE ORGANS AND BREASTS
Numerous epidemiological studies have been performed on the incidence of breast, endometrial, ovarian, and cervical cancer in women using oral contraceptives. Although the risk of breast cancer may be slightly increased among current and recent users of combined oral contraceptives, this excess risk decreases over time after product discontinuation, and by 10 years after cessation the increased risk disappears. In addition, breast cancers diagnosed in current or ever-oral contraceptive users tend to be less invasive than in non-users.
The risk of breast cancer does not increase with duration of use, and no relationships have been found with dose or type of steroid. The patterns of risk are also similar regardless of a woman's reproductive history or her family breast cancer history. The sub-group for whom risk has been found to be significantly elevated is women who first used combined oral contraceptives before age 20, but because breast cancer is so rare at these young ages, the number of cases attributable to this early combined oral contraceptive use is extremely small.
Women who currently have or have had breast cancer should not use combined hormonal contraceptives because breast cancer is a hormonally sensitive tumor.
Long-term case-controlled surveillance of users of depot medroxyprogesterone acetate (DMPA) found slight or no increased overall risk of breast cancer. A pooled analysis from two case-control studies, the World Health Organization (WHO) Study and the New Zealand Study, reported the relative risk of breast cancer for women who had ever used DMPA as 1.1. Overall, there was no increase in risk with increasing duration of use of DMPA. The relative risk of breast cancer for women of all ages who had initiated use of DMPA within the previous 5 years was estimated to be 2.0.
The WHO Study, a component of the pooled analysis described above, showed an increased relative risk of 2.19 of breast cancer associated with use of DMPA in women whose first exposure to drug was within the previous 4 years and who were under 35 years of age. However, the overall relative risk for ever-users of DMPA was only 1.2.
Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
A statistically insignificant increase in relative risk estimates of invasive squamous-cell cervical cancer has been associated with the use of DMPA in women who were first exposed before the age of 35 years. The overall, non-significant relative rate of invasive squamous-cell cervical cancer in women who ever used DMPA was estimated to be 1.11. No trends in risk with duration of use or times since initial or most recent exposure were observed.
In spite of many studies of the relationship between oral contraceptive use and breast and cervical cancers, a cause and effect relationship has not been established. No long-term studies have been conducted with LUNELLE™ Monthly Contraceptive Injection to evaluate risk for carcinoma of the reproductive organs.
-
HEPATIC NEOPLASIA
Benign hepatic adenomas are associated with oral contraceptive use, although the incidence of benign tumors is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases per 100,000 cases for users, a risk that increases after 4 or more years of use. Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies from Britain have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) oral contraceptive users. However, these cancers are extremely rare in the United States and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users.
-
OCULAR LESIONS
There have been clinical case reports of retinal thrombosis associated with the use of oral contraceptives. LUNELLE™ Monthly Contraceptive Injection should be discontinued if there is unexplained partial or complete loss of vision, onset of proptosis or diplopia, papilledema, or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately.
-
HORMONAL CONTRACEPTIVE USE BEFORE OR DURING PREGNANCY
The use of hormonal contraceptives during pregnancy is not indicated.
Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. Studies also do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when oral contraceptives are taken inadvertently during early pregnancy.
Pregnancies occurring in women receiving injectable progestin-only contraceptives are uncommon. Neonates from unexpected pregnancies that occurred 1 to 2 months after injection of DMPA may be at an increased risk of low birth weight, which, in turn, is associated with an increased risk of neonatal death. A significant increase in incidence of polysyndactyly and chromosomal anomalies was observed among infants of users of DMPA, the former being most pronounced in women under 30 years of age. The unrelated nature of these defects, the lack of confirmation from other studies, the distant preconceptual exposure to DMPA, and the chance effects due to multiple statistical comparisons, make a causal association unlikely.
Neonates exposed to MPA in utero and followed to adolescence, showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
Several reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. The risk of hypospadias (five to eight per 1,000 male births in the general population) may be approximately doubled with exposure to these drugs. There are insufficient data to quantify the risk to exposed female fetuses, but because some of these drugs induce mild virilization of the external genitalia of the female fetus and because of the increased association of hypospadias in the male fetus, these drugs should be avoided during pregnancy.
Unexpected pregnancies occurring in women receiving LUNELLE™ Monthly Contraceptive Injection are uncommon and have not shown congenital malformations or other adverse events.
The administration of combined hormonal contraceptives, such as LUNELLE™ Monthly Contraceptive Injection, to induce withdrawal bleeding should not be used as a test for pregnancy. LUNELLE™ Monthly Contraceptive Injection should not be used during pregnancy to treat threatened or habitual abortion. It is recommended that for any patient who has missed two consecutive periods, pregnancy should be considered before initiating or continuing LUNELLE™ Monthly Contraceptive Injection. If the patient has exceeded the prescribed injection interval (>33 days) for LUNELLE™ Monthly Contraceptive Injection, the possibility of pregnancy should be ruled out before another injection is administered.
-
GALLBLADDER DISEASE
Combined hormonal contraceptives may worsen existing gallbladder disease and may accelerate the development of this disease in previously asymptomatic women. Women with a history of combined hormonal contraceptive-related cholestasis are more likely to have the condition recur with subsequent combined hormonal contraceptive use.
In a study of 782 women taking LUNELLE™ Monthly Contraceptive Injection for up to 15 cycles, cholecystitis and cholelithiasis were the only serious adverse events judged to be possibly related to the study drug. They were reported as an adverse event in five subjects, and three subjects required cholecystectomy.
-
CARBOHYDRATE AND LIPID METABOLIC EFFECTS
Combined hormonal or progestin-only contraceptives have been shown to cause glucose intolerance in some users. However, in the non-diabetic woman, combined hormonal contraceptives appear to have no effect on fasting blood glucose. Pre-diabetic and diabetic patients should be carefully observed while receiving therapy with LUNELLE™ Monthly Contraceptive Injection.
A small proportion of women may have persistent hypertriglyceridemia while using oral contraceptives. Changes in serum triglycerides and lipoprotein levels have been reported in oral contraceptive users.
-
ELEVATED BLOOD PRESSURE
An increase in blood pressure has been reported in women taking oral contraceptives and this increase is more likely in older oral contraceptive users and with continued use. Data from the Royal College of General Practitioners and subsequent randomized trials have shown that the incidence of hypertension increases with increasing concentrations of progestogens. In a US clinical study, no increase in mean blood pressure was observed over 15 months use of LUNELLE™ Monthly Contraceptive Injection.
Women with a history of hypertension or hypertension-related diseases, or renal disease should be encouraged to use another method of contraception. If women elect to use combined hormonal contraceptives such as LUNELLE™ Monthly Contraceptive Injection, they should be monitored closely and if significant elevation of blood pressure occurs, LUNELLE™ Monthly Contraceptive Injection should be discontinued. For most women, elevated blood pressure will return to normal after stopping oral contraceptives, and there is no difference in the occurrence of hypertension among former and never-users.
-
HEADACHE
The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent, or severe requires evaluation of the cause before further injections of LUNELLE™ Monthly Contraceptive Injection are given.
-
BLEEDING IRREGULARITIES
Most women using LUNELLE™ Monthly Contraceptive Injection (58.6%) experienced alteration of menstrual bleeding patterns, including 4.1% amenorrhea, after one year of use. Altered bleeding patterns include frequent bleeding, irregular bleeding, prolonged bleeding, infrequent bleeding, and amenorrhea. As women continued using LUNELLE™ Monthly Contraceptive Injection, the percent experiencing frequent or prolonged bleeding decreased, while the percent experiencing amenorrhea increased. The percent of women experiencing irregular bleeding remained fairly constant at approximately 30% throughout the first year of use.
Regardless of the bleeding pattern, subsequent injections should be given 1 month (28 to 30 days, not to exceed 33 days) after the previous injection, unless discontinuation is medically indicated.
If abnormal bleeding associated with LUNELLE™ Monthly Contraceptive Injection persists or is severe, appropriate investigation should be instituted to rule out the possibility of organic pathology, and appropriate treatment should be instituted when necessary. In the event of amenorrhea, pregnancy should be ruled out.
-
BONE MINERAL DENSITY CHANGES
Use of injectable progestogen-only methods may be considered among the risk factors for development of osteoporosis. The rate of bone loss is greatest in the early years of use and then subsequently approaches the normal rate of age-related fall. Formal studies on the effect of bone mineral density changes in women receiving LUNELLE™ Monthly Contraceptive Injection have not been conducted.
-
ANAPHYLAXIS AND ANAPHYLACTOID REACTION
Anaphylaxis and anaphylactoid reactions have been reported with the components of LUNELLE™ Monthly Contraceptive Injection. Allergic reactions occurring in women using LUNELLE™ Monthly Contraceptive Injection have been mainly dermatologic, not respiratory, in nature. If an anaphylactic reaction occurs, appropriate therapy should be instituted. Serious anaphylactic reactions require emergency medical treatment.
PRECAUTIONS
-
General. Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
-
Physical Examination.
It is good medical practice for all women to have an annual history and physical examination, including women using combined hormonal contraceptives. The physical examination should include special reference to blood pressure, breasts, abdomen and pelvic organs, including cervical cytology, and relevant laboratory tests. In case of undiagnosed, persistent, or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
-
Weight Change.
In a study of 782 women using LUNELLE™ Monthly Contraceptive Injection for up to 15 cycles, 5.7% of participants discontinued due to weight gain. Weight gain was the most common adverse event leading to discontinuation of the drug. Women gained an average of 4 pounds during the first year, and an additional 2 pounds during the second year, of LUNELLE™ Monthly Contraceptive Injection use. The range of weight change during the first year of LUNELLE™ Monthly Contraceptive Injection use was 48 pounds lost to 49 pounds gained. The following table shows the range of weight changes seen for women continuing use up to 24 cycles.
Weight
Change |
12 Cycles
(n=469)
|
15 Cycles
(n=433)
|
24
Cycles
(n=111)
|
|
Lost >20 pounds
|
1%
|
2%
|
5%
|
|
Lost >10 to 20 pounds
|
6%
|
6%
|
7%
|
|
Gained >10 to 20 pounds
|
19%
|
24%
|
14%
|
|
Gained >20 pounds
|
5%
|
7%
|
23%
|
|
-
Lipid Disorders.
Women who are being treated for hyperlipidemias should be followed closely if they use combined hormonal contraceptives. Some progestogens may elevate LDL levels and may render the control of hyperlipidemias more difficult.
-
Liver Function.
If jaundice develops in any woman receiving combined hormonal contraceptives, the medication should be discontinued. Steroid hormones may be poorly metabolized in patients with impaired liver function.
-
Fluid Retention.
Progestogens and/or estrogens may cause some degree of fluid retention; therefore, caution should be used in treating any patient with a pre-existing medical condition that might be adversely affected by fluid retention.
-
Contact Lenses.
Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
-
Emotional Disorders.
Patients becoming significantly depressed while taking combined hormonal contraceptives should stop the medication and use an alternative method of contraception in an attempt to determine whether the symptom is drug-related. Women with a history of depression should be carefully observed and consideration should be given to the discontinuation of LUNELLE™ Monthly Contraceptive Injection if depression recurs to a serious degree.
-
Effects of Other Drugs on MPA
Aminoglutethamide may decrease the serum concentration of MPA. Users of LUNELLE™ Monthly Contraceptive Injection should be informed of the possibility of decreased effectiveness with the use of this or any related drug. (See CLINICAL PHARMACOLOGY , Drug-Drug Interactions .)
-
Effects of Other Drugs on Combined Hormonal Contraceptives
Rifampin
Metabolism of some synthetic estrogens (e.g., ethinyl estradiol) and progestins (e.g., norethindrone) is increased by rifampin. A reduction in contraceptive effectiveness and an increase in menstrual irregularities have been associated with concomitant use of rifampin.
Anticonvulsants.
Anticonvulsants such as phenobarbital, phenytoin, and carbamazepine have been shown to increase the metabolism of some synthetic estrogens and progestins, which could result in a reduction of contraceptive effectiveness.
Antibiotics.
Pregnancy while taking oral contraceptives has been reported when the oral contraceptives were administered with antimicrobials such as ampicillin, tetracycline, and griseofulvin. However, clinical pharmacokinetic studies have not demonstrated any consistent effects of antibiotics (other than rifampin) on plasma concentrations of synthetic steroids.
Herbal products.
Herbal products containing St. John's Wort (hypericum perforatum) may induce hepatic enzymes (cytochrome P450) and p-glycoprotein transporter and may reduce the effectiveness of contraceptive steroids. This may also result in breakthrough bleeding.
Other.
Ascorbic acid and acetominophen may increase plasma concentrations of some synthetic estrogens, possibly by inhibition of conjugation. A reduction in contraceptive effectiveness and an increased incidence of menstrual irregularities has been suggested with phenylbutazone.
-
Effects of Combined Hormonal Contraceptives on Other Drugs
Combined hormonal contraceptives containing some synthetic estrogens (e.g., ethinyl estradiol) may inhibit the metabolism of other compounds. Increased plasma concentrations of cyclosporine, prednisolone and theophylline have been reported with concomitant administration of oral contraceptives. In addition, oral contraceptives may induce the conjugation of other compounds. Decreased plasma concentrations of acetominophen and increased clearance of temazepam, salicylic acid, morphine and clofibric acid have been noted when these drugs were administered with oral contraceptives.
-
Drug Interactions with Laboratory Tests
Certain endocrine and liver function tests and blood components may be affected by combined hormonal contraceptives:
-
Increased prothrombin and factors VII, VIII, IX, and X; decreased antithrombin 3; increased norepinephrine-induced platelet aggregability.
-
Increased thyroid binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI). T4 by column or by radioimmunoassay. Free T3 resin uptake is decreased, reflecting the elevated TBG, free T4 concentration is unaltered.
-
Other binding proteins may be elevated in serum.
-
Sex-hormone-binding-globulins are increased and result in elevated levels of total circulating sex steroids and corticoids; however, free or biologically active levels remain unchanged.
-
Triglycerides may be increased.
-
Glucose tolerance may be decreased.
-
Serum folate levels may be depressed by combined hormonal contraceptive therapy. This may be of clinical significance if a woman becomes pregnant shortly after discontinuing combined hormonal contraceptives.
The pathologist should be advised of progestogen and estrogen therapy when relevant tissue specimens are submitted.
The following laboratory tests may be affected by progestins including LUNELLE™ Monthly Contraceptive Injection:
-
Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
-
Gonadotropin levels are decreased.
-
Sex-hormone-binding-globulin concentrations are decreased.
-
Sulfobromophthalein and other liver function test values may be increased.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
See WARNINGS section.
PREGNANCY
Pregnancy Category X. See CONTRAINDICATIONS and WARNINGS .
Return of Ovulation and Fertility
Ovulation (signaled by a rise in serum progesterone concentrations >/=4.7 ng/mL) was observed 63 to 112 days after the third monthly injection of LUNELLE™ Monthly Contraceptive Injection in 11 of 14 women participating in a pharmacodynamic study. The remaining three women had not ovulated by day 85 and were lost to follow-up.
In a study of 21 women who received LUNELLE™ Monthly Contraceptive Injection for 3 months, 52% ovulated during the first post-treatment month, and 71% during the second post-treatment month. In another study of 10 women receiving long-term administration (2 years of treatment) of LUNELLE™ Monthly Contraceptive Injection, 60% ovulated by the third post-treatment month.
A study of 70 women who discontinued LUNELLE™ Monthly Contraceptive Injection to become pregnant demonstrated that more than 50% achieved fertility within 6 months after discontinuation, and 83% did so by 1 year.
NURSING MOTHERS
The effects of LUNELLE™ Monthly Contraceptive Injection in nursing mothers have not been evaluated and are unknown. However, estrogen administration to nursing mothers has been shown to decrease the quantity and quality of breast milk. Small amounts of combined hormonal contraceptive steroids have been identified in the milk of nursing mothers and a few adverse effects on the child have been reported, including jaundice and breast enlargement. Long-term follow-up of children whose mothers used combined hormonal contraceptives while breastfeeding has shown no deleterious effects. However, women who are breastfeeding should not start taking combined hormonal contraceptives until six weeks postpartum.
PEDIATRIC USE
Safety and efficacy of LUNELLE™ Monthly Contraceptive Injection have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under 16 years of age and users 16 years of age and older. Use of this product before menarche is not indicated.
INFORMATION FOR PATIENTS
Patients should be given a copy of the patient labeling prior to administration of LUNELLE™ Monthly Contraceptive Injection.
Patients should be advised that the contraceptive efficacy of LUNELLE™ Monthly Contraceptive Injection depends on receiving injections monthly (28 to 30 days, not to exceed 33 days). The injection schedule must be measured by the number of days, not by bleeding episodes. It is recommended that for any patient who has missed two consecutive menstrual periods, pregnancy should be considered before initiating or continuing LUNELLE™ Monthly Contraceptive Injection. Thereafter, a woman who has continued amenorrhea while using LUNELLE™ Monthly Contraceptive Injection and who has received her injections according to the recommended dosing schedule may continue to receive subsequent injections each month after the previous injection (not to exceed 33 days), unless discontinuation is medically indicated. All patients presenting for a follow-up injection of LUNELLE™ Monthly Contraceptive Injection after day 33 should use a barrier method of contraception and should not receive another injection of
LUNELLE™ Monthly Contraceptive Injection until pregnancy has been ruled out.
Patients should be advised that menstrual bleeding patterns are likely to be disrupted with use of LUNELLE™ Monthly Contraceptive Injection. A few patients may experience amenorrhea. Irregular bleeding that occurs after a regular bleeding pattern has emerged should be investigated. In the presence of excessive or prolonged bleeding, other causes should be investigated and consideration should be given to alternative methods of contraception.
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
ADVERSE REACTIONS
An increased risk of the following serious adverse reactions has been associated with the use of combined hormonal contraceptives (see CONTRAINDICATIONS and WARNINGS ).
The following adverse reactions have been reported in patients receiving LUNELLE™ Monthly Contraceptive Injection and are believed to be drug-related:
There is evidence of an association between the following conditions and the use of combined hormonal contraceptives, although additional confirmatory studies are needed:
The following additional adverse reactions have been reported in users of combined hormonal contraceptives, and are believed to be drug-related:
The following additional adverse reactions have been reported in users of combined hormonal contraceptives, and the association has been neither confirmed nor refuted:
The most frequent adverse events (reported by 1% or more patients) leading to discontinuation in various trials of women using LUNELLE™ Monthly Contraceptive Injection were weight gain, menorrhagia, amenorrhea, metrorrhagia, vaginal spotting, emotional lability, acne, breast tenderness/pain, headache, dysmenorrhea, nausea, and depression.
OVERDOSAGE
Overdosage of a progestin/estrogen drug combination may cause nausea and vomiting, and vaginal bleeding or other menstrual irregularities in females.
DOSAGE AND ADMINISTRATION
LUNELLE™ Monthly Contraceptive Injection is effective for contraception during the first cycle of use when administered as recommended.
The recommended dose of LUNELLE™ Monthly Contraceptive Injection is 0.5 mL administered by intramuscular injection, into the deltoid, gluteus maximus, or anterior thigh. The aqueous suspension must be vigorously shaken just before use to ensure a uniform suspension of 25 mg medroxyprogesterone acetate and 5 mg estradiol cypionate.
First Injection
Second and Subsequent Injections
Switching from other Methods of Contraception
When switching from other contraceptive methods, LUNELLE™ Monthly Contraceptive Injection should be given in a manner that ensures continuous contraceptive coverage based upon the mechanism of action of both methods, e.g., patients switching from oral contraceptives should have their first injection of LUNELLE™ Monthly Contraceptive Injection within 7 days after taking their last active pill.
HOW SUPPLIED
LUNELLE™ Monthly Contraceptive Injection (25 mg medroxyprogesterone acetate and 5 mg estradiol cypionate per 0.5 mL sterile aqueous injectable suspension) is available in a vial containing enough product to deliver 0.5 mL for single-dose administration (NDC 0009-3484-04).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Rx
only
References available upon request.
Manufactured by:
Pharmacia & Upjohn Company
Kalamazoo, MI 49001, USA
October 2000 817 821 000
692804
3484-04
Every woman who considers using hormonal contraceptives must understand the benefits and risks of this type of birth control. This sheet contains important information about hormonal contraceptives that you need in order to decide if LUNELLE™ Monthly Contraceptive Injection is a good type of birth control for you. Please read this sheet carefully and ask your health care provider to help you compare LUNELLE™ Monthly Contraceptive Injection with other methods of birth control. This sheet is not meant to take the place of careful discussions with your health care provider. You should discuss the information provided in this sheet with him or her, both when you first start taking LUNELLE™ Monthly Contraceptive Injection and during your revisits. You should also follow your health care provider's advice with regard to regular check-ups while you are on LUNELLE™ Monthly Contraceptive Injection.
WHAT IS LUNELLE™ MONTHLY CONTRACEPTIVE INJECTION?
LUNELLE™ Monthly Contraceptive Injection is a type of hormonal birth control that is given as an injection (a shot) in your arm, thigh, or buttock once a month to prevent pregnancy. It contains hormones which have effects similar to the natural hormones, estrogen and progesterone, produced in your body. Similar combinations of hormones are found in some oral contraceptives also known as "birth control pills" or "the pill." When you receive your injections once a month as prescribed, LUNELLE™ Monthly Contraceptive Injection is as effective as birth control pills. When given according to the prescribed schedule, LUNELLE™ Monthly Contraceptive Injection is effective in preventing pregnancy during the cycle in which it is given. Clinical studies have shown that when women receive LUNELLE™ Monthly Contraceptive Injection according to the recommended schedule, the failure rate of this method of birth control is less than 1% per year.
The following table shows the typical failure rates for other methods of birth control during the first year of use:
Percentage of Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year: United States
|
|
% of Women
Experiencing
an Unintended
Pregnancy within
the First Year
of Use
|
% of
Women
Continuing
Use at
1 Year
3
|
|
|
Typical
Use
1
|
Perfect
Use
2
|
|
|
|
85
|
85
|
|
|
|
26
|
6
|
40
|
|
|
25
|
|
63
|
|
Calendar
|
|
9
|
|
|
|
|
3
|
|
|
|
|
2
|
|
|
Post-ovulation
|
|
1
|
|
|
|
|
|
|
|
Parous Women
|
40
|
26
|
42
|
|
Nulliparous Women
|
20
|
9
|
56
|
|
|
|
|
|
|
Parous Women
|
40
|
20
|
42
|
|
Nulliparous Women
|
20
|
9
|
56
|
|
|
20
|
6
|
56
|
|
|
19
|
4
|
|
|
|
|
|
|
|
Female (Reality)
|
21
|
5
|
56
|
|
Male
|
14
|
3
|
61
|
|
|
5
|
|
71
|
|
Progestin only
|
|
0.5
|
|
|
Combined
|
|
0.1
|
|
|
IUD
|
|
|
|
|
Progesterone T
|
2.0
|
1.5
|
81
|
|
Copper T 380A
|
0.8
|
0.6
|
78
|
|
LNg 20
|
0.1
|
0.1
|
81
|
|
Depo-Provera
|
0.3
|
0.3
|
70
|
|
Norplant and
|
|
|
|
|
Norplant-2
|
0.05
|
0.05
|
88
|
|
|
0.5
|
0.5
|
100
|
|
|
0.15
|
0.10
|
100
|
Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%.
9
Lactation Amenorrhea Method: LAM is a highly effective, temporary method of contraception.
10
|
Adapted from Hatcher et al., 1998.
|
1
Among
typical
couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
|
2
Among couples who initiate use of a method (not necessarily for the first time) and who use it
perfectly
(both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
|
3
Among couples attempting to avoid pregnancy, the percentage who continue to use a method for 1 year.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WHO SHOULD NOT TAKE LUNELLE™ MONTHLY CONTRACEPTIVE INJECTION
|
Cigarette smoking increases the risk of serious cardiovascular side effects from hormonal contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use hormonal contraceptives are strongly advised not to smoke.
|
Some women should not use hormonal contraceptives. For example, you should not take LUNELLE™ Monthly Contraceptive Injection if you are pregnant or think you may be pregnant. You should also not use LUNELLE™ Monthly Contraceptive Injection if you have any of the following conditions:
Tell your health care provider if you have ever had any of these conditions. Your health care provider can recommend a safer method of birth control.
OTHER CONSIDERATIONS BEFORE TAKING LUNELLE™ MONTHLY CONTRACEPTIVE INJECTION
For the majority of women, hormonal contraceptives can be taken safely. But there are some women who are at high risk of developing certain serious diseases that can be life-threatening or may cause temporary or permanent disability. Tell your health care provider if you have:
Women with any of these conditions should be checked often by their health care provider if they choose to use LUNELLE™ Monthly Contraceptive Injection.
Also, be sure to inform your doctor or health care provider if you smoke or are on any medications.
RISKS OF TAKING HORMONAL CONTRACEPTIVES
-
Risk of developing blood clots, heart attacks, and strokes
Blood clots and blockage of blood vessels are the most serious side effects of taking hormonal contraceptives. In particular, blood clots can occur in the legs and can travel to the lungs and can cause sudden blocking of the vessel carrying blood to the lungs. Rarely, clots occur in the blood vessels of the eye and may cause blindness, double vision, or impaired vision.
If you take hormonal contraceptives such as LUNELLE™ Monthly Contraceptive Injection and need elective surgery, need to stay in bed for a prolonged illness, or have recently had a baby, you may be at risk of developing blood clots. You should consult your doctor about stopping hormonal contraceptives three to four weeks before surgery and not taking hormonal contraceptives for two weeks after surgery or during bed rest. You should also not take hormonal contraceptives soon after delivery of a baby. It is advisable to wait for at least four weeks after delivery before using hormonal contraceptives such as LUNELLE™ Monthly Contraceptive Injection. (See also the section on Breast Feeding in GENERAL PRECAUTIONS .)
Hormonal contraceptives may also increase the tendency to develop strokes (stoppage or rupture of blood vessels in the brain) and angina pectoris and heart attacks (blockage of blood vessels in the heart). Any of these conditions can cause death or disability.
Smoking greatly increases the possibility of developing blood clots or suffering heart attacks and strokes. Furthermore, smoking and the use of hormonal contraceptives greatly increase the chances of developing and dying of heart disease, particularly if you are over 35 years of age.
-
Gallbladder disease
Hormonal contraceptive users probably have a greater risk than non-users of having gallbladder disease.
-
Liver tumors
In rare cases, hormonal contraceptives can cause benign but dangerous liver tumors. These benign liver tumors can rupture and cause fatal internal bleeding. In addition, a possible but not definite association has been found with hormonal contraceptives and liver cancers in two studies, in which a few women who developed these very rare cancers were found to have used hormonal contraceptives for long periods. However, liver cancers are extremely rare. The chance of developing liver cancer from using hormonal contraceptives is thus even rarer.
-
Cancer of the reproductive organs and breasts
There is, at present, no confirmed evidence that oral hormonal contraceptives increase the risk of cancer of the reproductive organs in human studies. Studies to date of women taking the pill have reported conflicting findings on whether pill use increases the risk of developing cancer of the breast. Most of the studies on breast cancer and pill use have found no overall increase in the risk of developing breast cancer, although some studies have reported an increased risk of developing breast cancer in certain groups of women.
Some studies have found an increase in the incidence of cancer of the cervix in women who use oral hormonal contraceptives. However, this finding may be related to factors other than the use of oral hormonal contraceptives.
Studies have found that women who used injectable hormonal contraceptives (Depo-Provera Contraceptive Injection) had no increased overall risk of developing cancer of the breast, ovary, uterus, or cervix. However, women under 35 years of age whose first exposure to Depo-Provera Contraceptive Injection was within the previous 4 to 5 years may have a slightly increased risk of developing breast cancer similar to that seen with oral contraceptives.
Women who use hormonal contraceptives and have a strong family history of breast cancer or who have breast nodules or abnormal mammogram should be closely followed by their doctors.
-
Changes in bone mineral density
Use of injectable hormonal contraceptives containing the progesterone-type hormone found in LUNELLE™ Monthly Contraceptive Injection may be associated with a decrease in the amount of mineral stored in your bones. This could increase your risk of developing bone fractures. The rate of bone mineral loss is greatest in the early years of use of this type of contraceptive, but after that, it begins to resemble the normal rate of age-related bone mineral loss.
Formal studies on the effect of bone mineral density changes in women receiving LUNELLE™ Monthly Contraceptive Injection have not been conducted.
-
Allergic reactions
Severe allergic reactions have been reported in some women using injectable hormonal contraceptives containing the progesterone-type hormone found in LUNELLE™ Monthly Contraceptive Injection. Allergic reactions occurring in women using LUNELLE™ Monthly Contraceptive Injection have been mainly skin reactions, and not respiratory in nature. Serious allergic reactions require emergency medical treatment.
ESTIMATED RISK OF DEATH FROM A BIRTH CONTROL METHOD OR PREGNANCY
All methods of birth control and pregnancy are associated with a risk of developing certain diseases that may lead to disability or death. An estimate of the number of deaths associated with different methods of birth control and pregnancy has been calculated and is shown in the following table.
Annual Number of BIrth-Related or Method-Related Deaths Associated with Control of Fertility per 100,000 Non-sterile Women, by Fertility Control Method According to Age
|
|
Range of Ages (years)
|
|
15-19
|
20-24
|
25-29
|
30-34
|
35-39
|
40-44
|
|
|
7.0
|
7.4
|
9.1
|
14.8
|
25.7
|
28.2
|
Oral hormonal contraceptives **
(non-smoker)
|
0.3
|
0.5
|
0.9
|
1.9
|
13.8
|
31.6
|
Oral hormonal contraceptives **
(smoker)
|
2.2
|
3.4
|
6.6
|
13.5
|
51.1
|
117.2
|
|
|
0.8
|
0.8
|
1.0
|
1.0
|
1.4
|
1.4
|
|
|
1.1
|
1.6
|
0.7
|
0.2
|
0.3
|
0.4
|
|
|
1.9
|
1.2
|
1.2
|
1.3
|
2.2
|
2.8
|
|
|
2.5
|
1.6
|
1.6
|
1.7
|
2.9
|
3.6
|
* Deaths are birth-related
|
|
|
|
In the above table, the risk of death from any birth control method is less than the risk of childbirth, except for oral hormonal contraceptive users over the age of 35 who smoke and oral hormonal contraceptive users over the age of 40 even if they do not smoke. It can be seen in the table that for women aged 15 to 39, the risk of death was highest with pregnancy (7-26 deaths per 100,000 women, depending on age). Among oral hormonal contraceptive users who do not smoke, the risk of death is always lower than that associated with pregnancy for any age group, although over the age of 40, the risk increases to 32 deaths per 100,000 women, compared to 28 associated with pregnancy at that age. However, for oral hormonal contraceptive users who smoke and are over the age of 35, the estimated number of deaths exceeds those for other methods of birth control. If a woman is over the age of 40 and smokes, her estimated risk of death is four times higher (117/100,000 women) than the estimated risk associated with
pregnancy (28/100,000 women) in that age group.
An Advisory Committee of the FDA discussed this issue in 1989 and recommended that the benefits of oral contraceptive use by healthy, non-smoking women over 40 years of age may outweigh the possible risks. However, women of all ages are cautioned to use the lowest dose oral contraceptive that is effective, and are strongly advised not to smoke.
WARNING SIGNALS
If any of these adverse effects occur while you are taking LUNELLE™ Monthly Contraceptive Injection, call your doctor immediately:
-
Sharp chest pain, coughing of blood, or sudden shortness of breath (indicating a possible clot in the lung)
-
Pain in the calf (indicating a possible clot in the leg)
-
Crushing chest pain or heaviness in the chest (indicating a possible heart attack)
-
Sudden severe headache or vomiting, dizziness or fainting, disturbances of vision or speech, weakness, or numbness in an arm or leg (indicating a possible stroke)
-
Sudden partial or complete loss of vision (indicating a possible clot in the eye)
-
Breast lumps (indicating possible breast cancer or fibrocystic disease of the breast; ask your doctor or health care provider to show you how to examine your breasts)
-
Severe pain or tenderness in the abdominal area (indicating a possibly ruptured liver tumor, ovarian cyst, or pregnancy outside the uterus)
-
Difficulty in sleeping, weakness, lack of energy, fatigue, or change in mood (possibly indicating severe depression)
-
Jaundice or a yellowing of the skin or eyeballs, accompanied frequently by fever, fatigue, loss of appetitie, dark-colored urine, or light-colored bowel movements (indicating possible liver problems)
-
Persistent pain, pus, or bleeding at the injection site
-
Unusually heavy vaginal bleeding
SIDE EFFECTS OF LUNELLE™ MONTHLY CONTRACEPTIVE INJECTION
-
Vaginal bleeding
Most women using LUNELLE™ Monthly Contraceptive Injection experience alteration of menstrual bleeding. Bleeding patterns may vary from a single monthly bleed to no bleeding at all or slight staining between menstrual periods to frequent, prolonged, and/or unpredictable bleeding. In any given injection interval, approximately 50% of women using LUNELLE™ Monthly Contraceptive Injection experience withdrawal bleeding that begins 20-25 days after the injection. Withdrawal bleeding lasts more than 7 days in 42% of women during the first month of use and in 29% of women at the end of one year of use. In any given injection interval, approximately 15% of women may have no bleeding at all and 10% may experience bleeding or spotting at various times in the cycle. Irregular bleeding often occurs during the first few months of LUNELLE™ Monthly Contraceptive Injection use and may persist with continued use in up to one third of women. Your menstrual blood flow may be heavier or lighter, and there may be no
bleeding, fewer days of bleeding, or more days of bleeding than what you have previously experienced. Such bleeding usually does not indicate any serious problems. If an altered bleeding pattern persists or the bleeding is severe, discuss it with your health care provider. There is also a small risk that (painful) cramps may be associated with bleeding.
-
Weight change
Weight gain is a common side effect in women using LUNELLE™ Monthly Contraceptive Injection. The average expected weight gain is 4 pounds in the first year of use. Some women gain more than 10 to 20 pounds in the first year. Women have gained as much as 49 pounds or lost as much as 48 pounds in one year of use. Clinical trials showed wide variability in individual weight change with an increasing percentage of LUNELLE™ Monthly Contraceptive Injection users experiencing weight change in excess of 10 to 20 pounds with continued treatment.
-
Contact lenses
If you wear contact lenses and notice a change in vision or an inability to wear your lenses, contact your doctor or health care provider.
-
Fluid retention
Hormonal contraceptives may cause edema (fluid retention) with swelling of the fingers or ankles and may raise your blood pressure. If you experience fluid retention, contact your doctor or health care provider.
-
Other side effects
Other side effects may include breast pain or tenderness, acne, change in appetite, nausea, headache, nervousness, depression, mood changes, changes in sexual desire, dizziness, loss of scalp hair, rash, and vaginal infections.
If any of these side effects bother you, call your health care provider.
GENERAL PRECAUTIONS
-
Missed periods and use of hormonal contraceptives before or during early pregnancy.
You may not menstruate regularly after you receive an injection of LUNELLE™ Monthly Contraceptive Injection. If you have received your injections regularly and miss one menstrual period, be sure to inform your health care provider. The risk of unexpected pregnancy for women receiving injectable contraceptives as scheduled is very low. If you have not received your injections as scheduled and missed a menstrual period, or if you missed two consecutive menstrual periods, you may be pregnant. Check with your health care provider immediately to determine whether you are pregnant. Do not continue the injections until you are sure you are not pregnant, but use another method of contraception.
There is no conclusive evidence that oral hormonal contraceptive use is associated with an increase in birth defects, when taken inadvertently during early pregnancy. Nevertheless, hormonal contraceptives should not be used during pregnancy.
With Depo-Provera Contraceptive Injection, there have been reports of an increased risk of low birth weight and neonatal infant death or other health problems in infants conceived close to the time of injection. However, these pregnancies are uncommon. Children exposed in the womb to one of the hormones found in LUNELLE™ Monthly Contraceptive Injection (MPA), and followed to adolescence, showed no evidence of any adverse effects on their health including their physical, mental, sexual or social development.
If you think you may have become pregnant while using LUNELLE™ Monthly Contraceptive Injection, see your health care provider as soon as possible. You should check with your health care provider about risks to your unborn child from any medication taken during pregnancy.
-
While breast feeding
If you are breast feeding, consult your health care provider before starting hormonal contraceptives, including LUNELLE™ Monthly Contraceptive Injection. Some of the drugs in hormonal contraceptives are passed on to the child in breast milk. A few adverse effects on the child have been reported, including yellowing of the skin (jaundice) and breast enlargement. In addition, hormonal contraceptives may decrease the amount and quality of your milk. To insure the best quantity and quality of your breast milk, you should wait until 6 weeks after childbirth before you start using LUNELLE™ Monthly Contraceptive Injection. If possible, do not use hormonal contraceptives while breast feeding.
Breast feeding provides only partial protection from becoming pregnant and this partial protection decreases significantly as you breast feed for longer periods of time. You should use another method of contraception while breast feeding and consider starting hormonal contraceptives only after you have weaned your child completely.
-
Laboratory tests
If you are scheduled for any laboratory tests, tell your doctor you are taking a hormonal contraceptive. Certain blood tests may be affected by hormonal contraceptives.
-
Drug interactions
Certain drugs may interact with hormonal contraceptives to make them less effective in preventing pregnancy or cause a change in bleeding patterns. Such drugs include aminoglutethimide, rifampin, drugs used for epilepsy such as barbiturates (for example, phenobarbital), carbamazepine, and phenytoin (Dilantin is one brand of this drug), phenylbutazone (Butazolidin is one brand), herbal products containing St. John's Wort (hypericum perforatum), and possible certain antibiotics. You may need to use an additional contraception method when you take drugs which can make hormonal contraceptives less effective. Drug interaction studies have not been conducted with LUNELLE™ Monthly Contraceptive Injection.
-
Sexually transmitted diseases
This product (like all hormonal contraceptives) is intended to prevent pregnancy. It does not protect against transmission of HIV (AIDS) and other sexually transmitted diseases such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
-
Weight change
LUNELLE™ Monthly Contraceptive Injection may cause weight gain of more than 10 pounds.
WHEN DO I GET MY LUNELLE™ MONTHLY CONTRACEPTIVE INJECTION?
LUNELLE™ Monthly Contraceptive Injection can only be effective if you receive your injections at the proper times.
First Injection
Next Injections
What Happens if I Miss an Injection or Wait Longer than 33 Days Between Injections?
Pregnancy Due to Failure with LUNELLE™ Monthly Contraceptive Injection
The incidence of failure with LUNELLE™ Monthly Contraceptive Injection in pregnancy is less than 1 percent (i.e., one pregnancy per 100 women per year) if given every month as directed. If you think that you may be pregnant, be sure to call your health care provider.
What If I Want to Become Pregnant?
You will need to stop your monthly injections of LUNELLE™ Monthly Contraceptive Injection. Most women begin to produce eggs again (and could become pregnant) about two to three months after their last injection.
There may be some delay in becoming pregnant after you stop using hormonal contraceptives, including LUNELLE™ Monthly Contraceptive Injection, especially if you had irregular menstrual cycles before you started using hormonal contraceptives. There does not appear to be any increase in birth defects in newborn babies when pregnancy occurs soon after stopping hormonal contraceptives.
OVERDOSAGE
Serious ill effects have not been reported following ingestion of large doses of oral hormonal contraceptives by young children. Overdosage may cause nausea and withdrawal bleeding in females. In case of overdosage, contact your health care provider or pharmacist.
OTHER INFORMATION
Your health care provider will take a medical and family history before prescribing hormonal contraceptives. You should receive yearly physical examinations by your health care provider. Be sure to inform your health care provider if there is a family history of any of the conditions listed previously in this leaflet. Be sure to keep all appointments with your health care provider, because this is a time to determine if there are early signs of side effects of hormonal contraceptive use. If you want more information about hormonal contraceptives, ask your health care provider or pharmacist for a more technical leaflet called the Prescribing Information that you may wish to read.
Each 0.5 mL dose of LUNELLE™ Monthly Contraceptive Injection contains:
Active Ingredients: medroxyprogesterone acetate (25 mg), estradiol cypionate (5 mg)
Inactive ingredients: methylparaben (0.9 mg), polyethylene glycol (14.28 mg), polysorbate 80 (0.95 mg), propylparaben (0.1 mg), sodium chloride (4.28 mg), sterile water for injection
Manufactured by:
Pharmacia & Upjohn Company
Kalamazoo, MI 49001, USA
October 2000 817 821 000
692804
3484-04
Copyright© 2001 Medical Economics